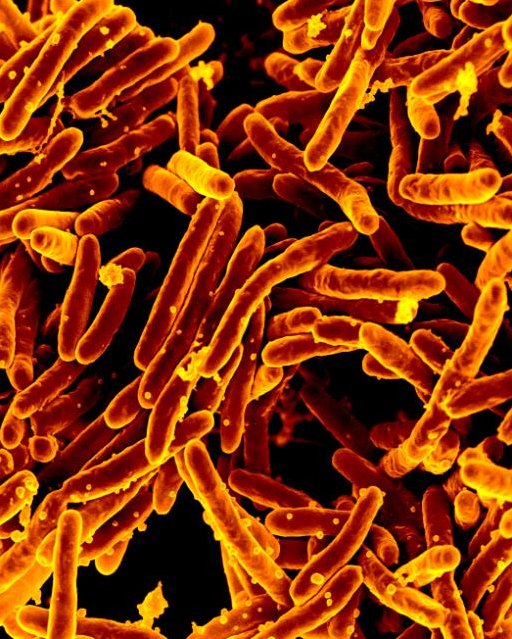
For the second time, contaminated bone graft products from the medical company Aziyo Biologics Inc. are linked to a highly unusual and deadly outbreak of tuberculosis.
This week, three new tuberculosis cases were identified, bringing the outbreak total to five, according to Politico. One person has died. The contaminated material, used for surgical and dental procedures, was implanted in at least 36 other patients, who are now being treated as if they have tuberculosis.
Aziyo Biologics issued a recall of all of its bone matrix products earlier this month "out of an abundance of caution" after the first two cases were identified. The Centers for Disease Control and Prevention reports that all unused products from the affected lot have been sequestered so that they will not be used. The affected materials had been sent to health care facilities in California, Michigan, New York, Oregon, Texas, and Virginia.
A representative for Aziyo did not immediately respond to Ars' request for comment. In the recall announcement, Aziyos CEO Randy Mills said: "We are taking immediate action to safeguard patients by implementing a full product recall as we work with the CDC to investigate this event. The people of Aziyo care deeply about the patients we serve and will continue to work with the medical community, patients, and regulatory authorities as we gather additional information."
Tuberculosis outbreaks linked to transplanted tissue are very rare, yet this isn't the first time Aziyo's bone graft products have been linked to such an outbreak. Just two years ago, a different bone graft product from the company was linked to a large outbreak in which at least 87 patients developed tuberculosis, and eight died.
That outbreak, which came to light in May 2021, was linked to contaminated product from a single tissue donor, a deceased man in his 80s. An outbreak investigation published last year found that the donor had "unrecognized risk factors, symptoms, and signs consistent with tuberculosis."
Tissue from the man was made into 154 units of bone matrix material—described as a malleable putty that includes human cells—which was shipped to 37 health care facilities in 20 US states. From there, 136 units were implanted into the spines of 113 patients, some of whom got multiple units. Of those 113 patients, 87 had signs of tuberculosis disease, 55 were readmitted to the hospital for complications, and 48 had to undergo secondary spinal surgeries for drainage, to remove damaged tissue, and/or to remove hardware. Of the 105 surviving patients, all were treated for tuberculosis, taking multiple antibiotics for a median course of 69 days.
It's unclear how the problem occurred again. In its recall announcement earlier this month, Aziyo said the contaminated lot, again from a single donor, had tested negative for the bacterium that causes tuberculosis, Mycobacterium tuberculosis, by an independent laboratory using a nucleic acid test before distribution. The company added that it is "fully cooperating and investigating the events" in coordination with the CDC and the Food and Drug Administration.
reader comments
64 with